U.S. cattle ranchers will soon have a new way to protect their dwindling herds from the threat of the parasite screwworm, which is decimating cattle in Mexico.
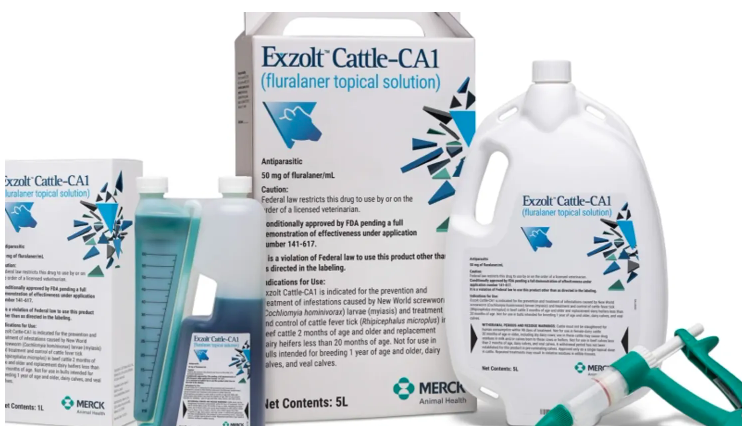
The U.S. Food and Drug Administration granted conditional approval for a drug called EXZOLT CATTLE-CA1, a topical treatment from Merck
Animal Health for the prevention and treatment of New World Screwworm. It can also be used as a treatment and control for the cattle fever tick.
Ranchers will have access to doses of the Merck drug starting on December 20 through their veterinarian.
“The conversation started in July with the FDA, and because there is an element of human food safety, there was a big data package we had to generate,” said Holger Lehmann, vice president of pharmaceutical research and development for Merck Animal Health. “This approval is a significant undertaking. The U.S. has received the product, and Mexico received it in early November, where it is being used,” Lehmann said.
Each dose is effective for 21 days before a new dose would need to be applied. The FDA has approved it with a 98-day withholding period to ensure no residue in meat.
Lehmann cautioned that the drug alone cannot eradicate the parasite any time soon. “Experts tell us in Mexico that they don’t expect that they’re going to be able to get rid of the screwworm problem quickly,” Lehmann said. “They think it’s a multi-year problem to get resolved.”
Screwworm is spread by hatching fly eggs in the open wounds of cattle, which feed on their living tissue. Humans can also get infected. To protect the U.S. cattle herd and to stop the spread of the parasitic fly, the U.S. Department of Agriculture has closed the border to Mexico for imports of live cattle, bison, and horses, on and off, since early 2025.